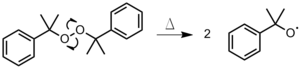
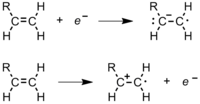
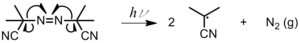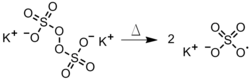Radical polymerization
Radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules.
Following its generation, the initiating radical adds (nonradical) monomer units, thereby growing the polymer chain.
Radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites.
The efficiency factor f is defined as the fraction of the original initiator which contributes to the polymerization reaction.
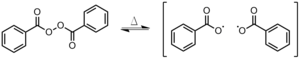
[8] In an ethene monomer, one electron pair is held securely between the two carbons in a sigma bond.
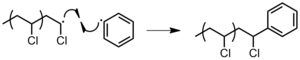
There may be anywhere from a few to thousands of propagation steps depending on several factors such as radical and chain reactivity, the solvent, and temperature.
However, this is very difficult to achieve and instead a pseudo-living polymerization occurs with only partial control of molecular weight and dispersity.
Under the steady-state approximation, the concentration of the active growing chains remains constant, i.e. the rates of initiation and of termination are equal.
In this case, the rate of chain propagation can be further described using a function of the initiator and monomer concentrations[20][21] The kinetic chain length v is a measure of the average number of monomer units reacting with an active center during its lifetime and is related to the molecular weight through the mechanism of the termination.
[22] Assuming no chain-transfer effect occurs in the reaction, the number average degree of polymerization Pn can be correlated with the kinetic chain length.
According to the thermodynamic equation ΔG = ΔH – TΔS, a negative enthalpy and an increasing entropy will shift the equilibrium towards polymerization.
In general, the polymerization is an exothermic process, i.e. negative enthalpy change, since addition of a monomer to the growing polymer chain involves the conversion of π bonds into σ bonds, or a ring–opening reaction that releases the ring tension in a cyclic monomer.
Meanwhile, during polymerization, a large amount of small molecules are associated, losing rotation and translational degrees of freedom.
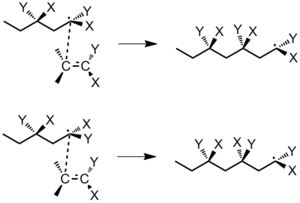
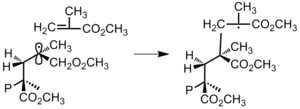
[24] The stereochemistry of polymerization is concerned with the difference in atom connectivity and spatial orientation in polymers that has the same chemical composition.
The major milestone in the stereochemistry was established by Ziegler and Natta and their coworkers in 1950s, as they developed metal based catalyst to synthesize stereoregular polymers.
[25] Atactic polymers consist of a random arrangement of stereochemistry and are amorphous (noncrystalline), soft materials with lower physical strength.
The Q–e scheme, the most widely used tool for the semi-quantitative prediction of monomer reactivity ratios, was first proposed by Alfrey and Price in 1947.
[26] The scheme takes into account the intrinsic thermodynamic stability and polar effects in the transition state.
[27] The polar effects in the transition state, the supposed permanent electric charge carried by that entity (radical or molecule), is quantified by the factor e, which is a constant for a given monomer, and has the same value for the radical derived from that specific monomer.
[28] Free radical polymerization has found applications including the manufacture of polystyrene, thermoplastic block copolymer elastomers,[29] cardiovascular stents,[30] chemical surfactants[31] and lubricants.
Block copolymers are used for a wide variety of applications including adhesives, footwear and toys.
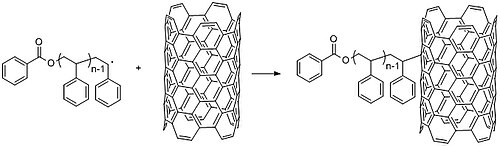
[32] CNTs intrinsic electronic properties lead them to form large aggregates in solution, precluding useful applications.
Adding small chemical groups to the walls of CNT can eliminate this propensity and tune the response to the surrounding environment.
[34] These gels are made of water-swellable nano-scale clay (especially those classed as smectites) enveloped by a network polymer.
Aqueous dispersions of clay are treated with an initiator and a catalyst and the organic monomer, generally an acrylamide.
[35] Free radical polymerization used in this context allows the synthesis of polymers from a wide variety of substrates (the chemistries of suitable clays vary).
Termination reactions unique to chain growth polymerization produce a material with flexibility, mechanical strength and biocompatibility.