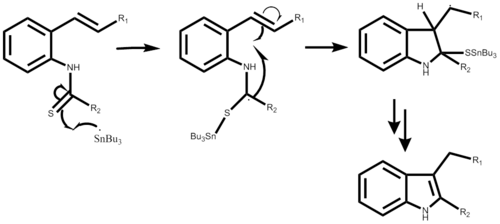
Fukuyama indole synthesis
[2] Most commonly tributyltin hydride is utilized as the reducing agent, with azobisisobutyronitrile (AIBN) as a radical initiator.
In addition the reaction is not stereospecific, in that both the cis and trans isoform can be used to obtain the desired product.
This reaction is a one-pot synthesis and results in yields ranging from 50% to 98% depending on the substituent.
The Fukuyama Indole synthesis can generate a range of different substituents at the 2,3 position that were previously unattainable without a protecting group on the nitrogen in the ring.
In addition, the fukuyama reaction plays a role in the syntheses of indolocarbazoles,[5] biindolyls,[5] and the total synthesis of vincadifformine and tabersonine.