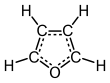
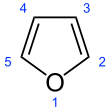
Furan
Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature.
Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse.
Furan itself was first prepared by Heinrich Limpricht in 1870, although he called it "tetraphenol" (as if it were a four-carbon analog to phenol, C6H5OH).
[8] It can also be prepared directly by thermal decomposition of pentose-containing materials, and cellulosic solids, especially pine wood.
[13] The molecule is flat but the C=C groups attached to oxygen retain significant double bond character.
The other lone pair of electrons of the oxygen atom extends in the plane of the flat ring system.
[23] Exposure to furan at doses about 2,000 times the projected level of human exposure from foods increases the risk of hepatocellular tumors in rats and mice and bile duct tumors in rats.