Galantamine
It is used clinically for treating early-stage Alzheimer's disease and memory impairments, although it has had limited success with the more advanced condition of dementia.
[6] Galantamine may cause serious adverse effects, such as stomach bleeding, liver injury or chest pain.
[15] Galantamine, sold under the brand name Razadyne among others, is indicated for the treatment of mild to moderate vascular dementia and Alzheimer's disease.
[6][7] The first person to extract galantamine and theorize its usefulness in medicine, was the Bulgarian chemist Dimitar Paskov in 1959.
In the United States, it is approved by the Food and Drug Administration (FDA) for the treatment of mild to moderate dementia.
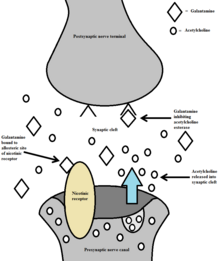
[8] Galantamine's competitive inhibition of acetylcholinesterase and allosteric nicotinic modulation serves as a dual mechanism of action.
[18] To reduce the prevalence of negative side effects associated with galantamine, such as nausea and vomiting, a dose-escalation scheme may be used.
[19] A dose-escalation scheme for Alzheimer's treatment involves a recommended starting dosage of 4 mg galantamine tablets given twice a day (8 mg/day).
[6] If treatment is interrupted for more than three days, the process is usually restarted, beginning at the starting dosage, and re-escalating to the current dose.
[6][23] Using galantamine may cause chest pain, bloody urine, stomach bleeding, and liver injury, among other side effects.
[6][25] By binding to the allosteric site of the nAChRs, a conformational change occurs which increases the receptors response to acetylcholine.
[26] However, recent studies suggest that Galantamine does not functionally act at human nAChRs α4β2 or α7 as a positive allosteric modulator.
Galantamine's effects on nAChRs and complementary acetylcholinesterase inhibition make up a dual mechanism of action.
Peak effect of inhibiting acetylcholinesterase was achieved about one hour after a single oral dose of 8 mg in some healthy volunteers.
[33] The effects on the central nervous system include anxiety, restlessness, confusion, ataxia, tremors, seizures, cardiorespiratory paralysis, and coma.
[33] Research supported in part by the US Army has led to a US patent application for the use of galantamine and/or its derivatives for treatment of organophosphate poisoning.
Galantamine was studied in the research cited in the patent application for use along with the well-recognized nerve agent antidote atropine.
[34] Galantamine given in addition to risperidone to autistic children has been shown to improve some of the symptoms of autism such as irritability, lethargy, and social withdrawal.
[36] As such, it is hypothesized that galantamine's dual action mechanism might have a similar effect in treating autistic children and adolescents.