Galvanic corrosion
A similar galvanic reaction is exploited in primary cells to generate a useful electrical voltage to power portable devices.
The electropotential difference between the reactions at the two electrodes is the driving force for an accelerated attack on the anode metal, which dissolves into the electrolyte.
The presence of an electrolyte and an electrical conducting path between the metals is essential for galvanic corrosion to occur.
Acidity or alkalinity (pH) is also a major consideration with regard to closed loop bimetallic circulating systems.
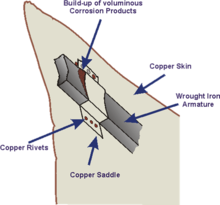
Although the problem had been anticipated when the structure was built by Gustave Eiffel to Frédéric Bartholdi's design in the 1880s, the insulation layer of shellac between the two metals had failed over time and resulted in rusting of the iron supports.
The structure was far from unsafe owing to the large number of unaffected connections, but it was regarded as a precautionary measure to preserve a national symbol of the United States.
[7][8] The problem recurred when vessels were sheathed in copper to reduce marine weed accumulation and protect against shipworm.
The copper sheathing had been delivered to the dockyard wrapped in the paper which was not always removed before the sheets were nailed to the hull.
[10][11] Serious galvanic corrosion has been reported on the latest US Navy attack littoral combat vessel the USS Independence caused by steel water jet propulsion systems attached to an aluminium hull.
[12] The unexpected fall in 2011 of a heavy light fixture from the ceiling of the Big Dig vehicular tunnel in Boston revealed that corrosion had weakened its support.
Improper use of aluminium in contact with stainless steel had caused rapid corrosion in the presence of salt water.
[13] The electrochemical potential difference between stainless steel and aluminium is in the range of 0.5 to 1.0 V, depending on the exact alloys involved, and can cause considerable corrosion within months under unfavorable conditions.
[17] There are several ways of reducing and preventing this form of corrosion: All metals can be classified into a galvanic series representing the electrical potential they develop in a given electrolyte against a standard reference electrode.
[19][page needed] When design considerations require that dissimilar metals come in contact, the difference in anodic index is often managed by finishes and plating.
[19][page needed] It will always be the metal with the most negative anodic index which will ultimately suffer from corrosion when galvanic incompatibility is in play.