Gas laws
[2] This experiment essentially paved the way towards the invention of the barometer, as well as drawing the attention of Robert Boyle, then a "skeptical" scientist working in England.
Later on, in 1676, the French physicist Edme Mariotte, independently arrived at the same conclusions of Boyle, while also noting some dependency of air volume on temperature.
[4] However it took another century and a half for the development of thermometry and recognition of the absolute zero temperature scale, which eventually allowed the discovery of temperature-dependent gas laws.
In 1662, Robert Boyle systematically studied the relationship between the volume and pressure of a fixed amount of gas at a constant temperature.
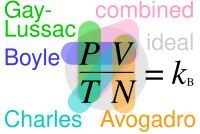
He observed that the volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature.
It states that, for a given mass of an ideal gas at constant pressure, the volume is directly proportional to its absolute temperature, assuming in a closed system.
The statement of Charles' law is as follows: the volume (V) of a given mass of a gas, at constant pressure (P), is directly proportional to its temperature (T).
However, the ideal gas law is a good approximation for most gases under moderate pressure and temperature.