Partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature.
[1] The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law).
This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood;[2] consequently, mixture ratios, like that of breathable 20% oxygen and 80% Nitrogen, are determined by volume instead of by weight or mass.
[3] Furthermore, the partial pressures of oxygen and carbon dioxide are important parameters in tests of arterial blood gases.
That said, these pressures can also be measured in, for example, cerebrospinal fluid.
[4][5] Examples: Dalton's law expresses the fact that the total pressure of a mixture of ideal gases is equal to the sum of the partial pressures of the individual gases in the mixture.
[6] This equality arises from the fact that in an ideal gas, the molecules are so far apart that they do not interact with each other.
Most actual real-world gases come very close to this ideal.
For example, given an ideal gas mixture of nitrogen (N2), hydrogen (H2) and ammonia (NH3):
[7] The ratio of partial pressures relies on the following isotherm relation:
It can be approximated both from partial pressure and molar fraction:[8]
It is a measure of the tendency of molecules and atoms to escape from a liquid or a solid.
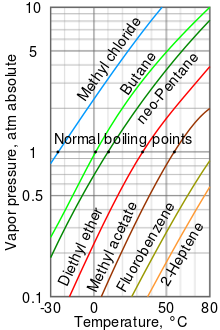
[9] As can be seen in the chart, the liquids with the highest vapor pressures have the lowest normal boiling points.
For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart.
It also has the lowest normal boiling point (−24.2 °C), which is where the vapor pressure curve of methyl chloride (the blue line) intersects the horizontal pressure line of one atmosphere (atm) of absolute vapor pressure.
At higher altitudes, the atmospheric pressure is less than that at sea level, so boiling points of liquids are reduced.
At the top of Mount Everest, the atmospheric pressure is approximately 0.333 atm, so by using the graph, the boiling point of diethyl ether would be approximately 7.5 °C versus 34.6 °C at sea level (1 atm).
It is possible to work out the equilibrium constant for a chemical reaction involving a mixture of gases given the partial pressure of each gas and the overall reaction formula.
For reversible reactions, changes in the total pressure, temperature or reactant concentrations will shift the equilibrium so as to favor either the right or left side of the reaction in accordance with Le Chatelier's Principle.
However, the reaction kinetics may either oppose or enhance the equilibrium shift.
Gases will dissolve in liquids to an extent that is determined by the equilibrium between the undissolved gas and the gas that has dissolved in the liquid (called the solvent).
Since both may be referred to as the Henry's law constant, readers of the technical literature must be quite careful to note which version of the Henry's law equation is being used.
Henry's law is an approximation that only applies for dilute, ideal solutions and for solutions where the liquid solvent does not react chemically with the gas being dissolved.
[14] Using diving terms, partial pressure is calculated as: For the component gas "i": For example, at 50 metres (164 ft) underwater, the total absolute pressure is 6 bar (600 kPa) (i.e., 1 bar of atmospheric pressure + 5 bar of water pressure) and the partial pressures of the main components of air, oxygen 21% by volume and nitrogen approximately 79% by volume are: The minimum safe lower limit for the partial pressures of oxygen in a breathing gas mixture for diving is 0.16 bars (16 kPa) absolute.
Hypoxia and sudden unconsciousness can become a problem with an oxygen partial pressure of less than 0.16 bar absolute.
The partial pressure of oxygen also determines the maximum operating depth of a gas mixture.
[14] Narcosis is a problem when breathing gases at high pressure.
Typically, the maximum total partial pressure of narcotic gases used when planning for technical diving may be around 4.5 bar absolute, based on an equivalent narcotic depth of 35 metres (115 ft).
A mixture which may be relatively safe at the surface could be dangerously toxic at the maximum depth of a dive, or a tolerable level of carbon dioxide in the breathing loop of a diving rebreather may become intolerable within seconds during descent when the partial pressure rapidly increases, and could lead to panic or incapacitation of the diver.
) are important parameters in tests of arterial blood gases, but can also be measured in, for example, cerebrospinal fluid.