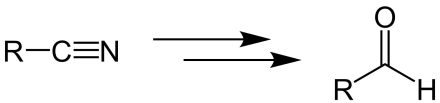
Stephen aldehyde synthesis
This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O).
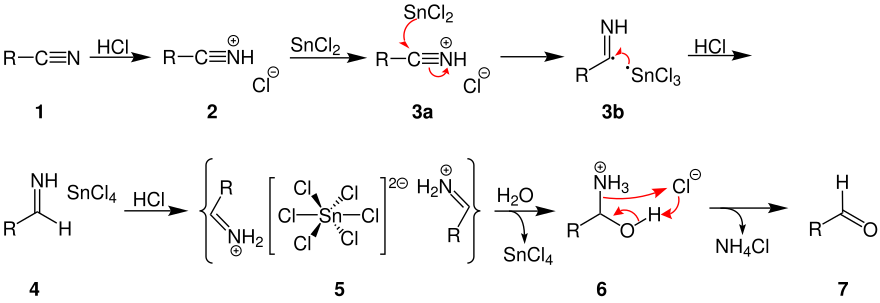
The following scheme shows the reaction mechanism: By addition of hydrogen chloride the used nitrile (1) reacts to its corresponding salt (2).
Substitutes that increase the electron density promote the formation of the aldimine-tin chloride adduct.
[4] In the past, the reaction was carried out by precipitating the aldimine-tin chloride, washing it with ether and then hydrolyzing it.
[5] In the Sonn-Müller method[6][7] the intermediate iminium salt is obtained from reaction of an amide PhCONHPh with phosphorus pentachloride.