Glycogen phosphorylase
Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation and allosteric effects.
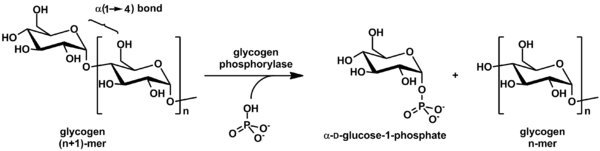
Although the reaction is reversible in vitro, within the cell the enzyme only works in the forward direction as shown below because the concentration of inorganic phosphate is much higher than that of glucose-1-phosphate.
Pyridoxal phosphate links with basic residues (in this case Lys680) and covalently forms a Schiff base.
Finally, the deprotonated inorganic phosphate acts as a nucleophile and bonds with the carbocation, resulting in the formation of glucose-1-phosphate and a glycogen chain shortened by one glucose molecule.
[3] The glycogen phosphorylase monomer is a large protein, composed of 842 amino acids with a mass of 97.434 kDa in muscle cells.
While the enzyme can exist as an inactive monomer or tetramer, it is biologically active as a dimer of two identical subunits.
[7] The allosteric site of AMP binding on muscle isoforms of glycogen phosphorylase are close to the subunit interface just like Ser14.
[8] AMP binding rotates the tower helices (residues 262-278) of the two subunits 50˚ relative to one another through greater organization and intersubunit interactions.
Residues 397-437 form this structure, which allows the protein to covalently bind to the glycogen chain a full 30 Å from the catalytic site .
In fact, 70% of dimeric phosphorylase in the cell exists as bound to glycogen granules rather than free floating.
[18][19] Hers' disease is often associated with mild symptoms normally limited to hypoglycemia, and is sometimes difficult to diagnose due to residual enzyme activity.
Hormones such as epinephrine, insulin and glucagon regulate glycogen phosphorylase using second messenger amplification systems linked to G proteins.
The increased calcium availability binds to the calmodulin subunit and activates glycogen phosphorylase kinase.
An increase in ATP concentration opposes this activation by displacing AMP from the nucleotide binding site, indicating sufficient energy stores.
As a result, PKA can no longer initiate the phosphorylation cascade that ends with formation of (active) glycogen phosphorylase a.
Overall, insulin signaling decreases glycogenolysis to preserve glycogen stores in the cell and triggers glycogenesis.