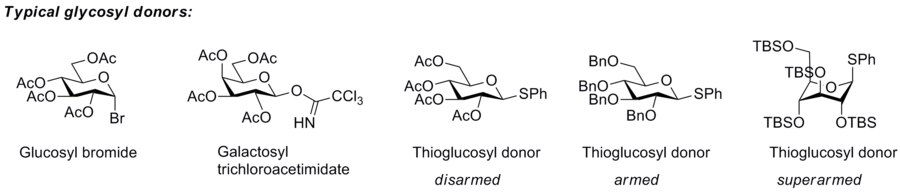
Glycosyl donor
By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new glycosidic bond.
More effective leaving groups are in general used in the glycosyl donors employed in chemical synthesis of glycosides.
[1] The so-called “armed-disarmed” principle The concept of armed and disarmed glycosyl donors refers to the increased reactivity of benzylated over benzoylated glycosyl donors, a phenomenon observed very early,[2] and which originates from the greater electron-withdrawing capability of ester blocking groups over ether blocking groups.
[3] This approach allowed him to carry out a one-pot synthesis of a trisaccharide by the n-pentenyl glycoside method.
[4] The concept has been extended to superarmed glycosyl donor by Mikael Bols and his collaborators.