Group 7 element
Like other groups, the members of this family show patterns in their electron configurations, especially the outermost shells resulting in trends in chemical behavior.
In nature, manganese is a fairly common element, whereas rhenium is rare, technetium only occurs in trace quantities, and bohrium is entirely synthetic.
The higher +7 oxidation state is more likely to exist in oxyanions, such as perbohrate, BhO4−, analogous to the lighter permanganate, pertechnetate, and perrhenate.
[6] Considerable research has centred on producing nanocrystalline Mn3O4 and various syntheses that involve oxidation of MnII or reduction of MnVI.
It adopts a centrosymmetric corner-shared bi-tetrahedral structure in which the terminal and bridging Tc−O bonds are 167pm and 184 pm respectively and the Tc−O−Tc angle is 180°.
Technetium halides exhibit different structure types, such as molecular octahedral complexes, extended chains, layered sheets, and metal clusters arranged in a three-dimensional network.
[6] The structures of these compounds often feature extensive Re-Re bonding, which is characteristic of this metal in oxidation states lower than VII.
[47] Dirhenium decacarbonyl can be oxidised with bromine to bromopentacarbonylrhenium(I):[48] Reduction of this pentacarbonyl with zinc and acetic acid gives pentacarbonylhydridorhenium:[49] Methylrhenium trioxide ("MTO"), CH3ReO3 is a volatile, colourless solid has been used as a catalyst in some laboratory experiments.
[64][65] The discovery of element 43 was finally confirmed in a 1937 experiment at the University of Palermo in Sicily by Carlo Perrier and Emilio Segrè.
[66] In mid-1936, Segrè visited the United States, first Columbia University in New York and then the Lawrence Berkeley National Laboratory in California.
Evidence of bohrium was first reported in 1976 by a Soviet research team led by Yuri Oganessian, in which targets of bismuth-209 and lead-208 were bombarded with accelerated nuclei of chromium-54 and manganese-55 respectively.
[69] In 1981, a German research team led by Peter Armbruster and Gottfried Münzenberg at the GSI Helmholtz Centre for Heavy Ion Research (GSI Helmholtzzentrum für Schwerionenforschung) in Darmstadt bombarded a target of bismuth-209 with accelerated nuclei of chromium-54 to produce five atoms of the isotope bohrium-262:[70] This discovery was further substantiated by their detailed measurements of the alpha decay chain of the produced bohrium atoms to previously known isotopes of fermium and californium.
[77] In South Africa, most identified deposits are located near Hotazel in the Northern Cape Province, with a 2011 estimate of 15 billion tons.
[78] Manganese is mainly mined in South Africa, Australia, China, Gabon, Brazil, India, Kazakhstan, Ghana, Ukraine and Malaysia.
[81] A more progressive extraction process involves directly reducing (a low grade) manganese ore in a heap leach.
Physical, chemical, and biological environmental impacts can occur due to this nodule mining disturbing the seafloor and causing sediment plumes to form.
This suspension includes metals and inorganic nutrients, which can lead to contamination of the near-bottom waters from dissolved toxic compounds.
[97] Chile has the world's largest rhenium reserves, part of the copper ore deposits, and was the leading producer as of 2005.
[105][106] The metal form is prepared by reducing ammonium perrhenate with hydrogen at high temperatures:[25] Bohrium is a synthetic element that does not occur in nature.
The facial isomer of both rhenium and manganese 2,2'-bipyridyl tricarbonyl halide complexes have been extensively researched as catalysts for electrochemical carbon dioxide reduction due to their high selectivity and stability.
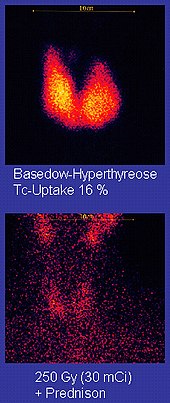
[113] The chemistry of technetium allows it to be bound to a variety of biochemical compounds, each of which determines how it is metabolized and deposited in the body, and this single isotope can be used for a multitude of diagnostic tests.
More than 50 common radiopharmaceuticals are based on technetium-99m for imaging and functional studies of the brain, heart muscle, thyroid, lungs, liver, gall bladder, kidneys, skeleton, blood, and tumors.
[115] The longer-lived isotope, technetium-95m with a half-life of 61 days, is used as a radioactive tracer to study the movement of technetium in the environment and in plant and animal systems.
[120] For this reason, pertechnetate has been used as an anodic corrosion inhibitor for steel, although technetium's radioactivity poses problems that limit this application to self-contained systems.
[120] The mechanism by which pertechnetate prevents corrosion is not well understood, but seems to involve the reversible formation of a thin surface layer (passivation).
[122] As noted, the radioactive nature of technetium (3 MBq/L at the concentrations required) makes this corrosion protection impractical in almost all situations.
Re(R-bpy)(CO)3X complexes exclusively produce CO from CO2 reduction with Faradaic efficiencies of close to 100% even in solutions with high concentrations of water or Brønsted acids.
[108] The catalytic mechanism of Re(R-bpy)(CO)3X involves reduction of the complex twice and loss of the X ligand to generate a five-coordinate active species which binds CO2.
The primary hazard when working with technetium is inhalation of dust; such radioactive contamination in the lungs can pose a significant cancer risk.
It is present as a coenzyme in biological processes that include macronutrient metabolism, bone formation, and free radical defense systems.