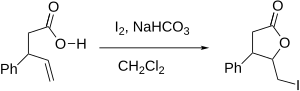
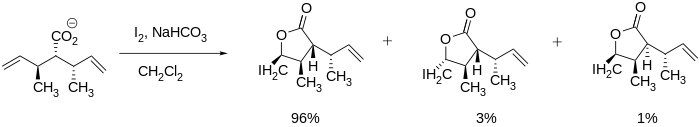
Iodolactonization
Iodolactonization (or, more generally, halolactonization) is an organic reaction that forms a ring (the lactone) by the addition of an oxygen and iodine across a carbon-carbon double bond.
Iodolactonization has been used in the synthesis of many natural products including those with medicinal applications such as vernolepin and vernomenin,[2] two compounds used in tumor growth inhibition, and vibralactone, a pancreatic lipase inhibitor.
The use of elemental chlorine is procedurally difficult because it is a gas at room temperature, and the electrophilic addition product can be rapidly produced as in bromolactonization.
[6] The reaction mechanism involves the formation of a positively charged halonium ion in a molecule that also contains a carboxylic acid (or other functional group that is a precursor to it).
This is due to the fact that the more substituted carbon is better able to maintain a partial positive charge and is thus more electrophilic and susceptible to nucleophilic attack.
Stereoselective iodolactonizations have been seen in literature and can be very useful in synthesizing large molecules such as the aforementioned vernopelin and vernomenin because the lactone can be formed while maintaining other stereocenters.
In 1977, Samuel Danishefsky and coworkers were able to synthesize the tumor growth inhibitors dl-vernolepin and dl-vernomenin via a multistep process in which a lactonization was employed.