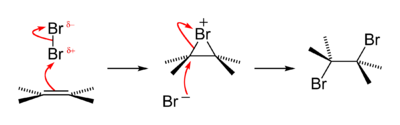Halogen addition reaction
In this way the two halogens add in an anti addition fashion, and when the alkene is part of a cycle the dibromide adopts the trans configuration.
Maleic acid with a cis-double bond forms the dibromide as a mixture of enantiomers: while the trans-isomer fumaric acid forms a single meso compound: The reaction is even stereospecific in alkenes with two bulky tert-butyl groups in a cis position as in the compound cis-di-tert-butylethylene.
In an alternative reaction scheme depicted below the reactive intermediate is a β-bromocarbocation or β-bromocarbonium ion with one of the carbon atoms a genuine carbocation.
In alkenes such as anetholes and stilbenes the substituents are able to stabilize the carbocation by donating electrons at the expense of the halonium ion.
In 1967 the group of George A. Olah obtained NMR spectra of tetramethylethylenebromonium ions by dissolving 2,3-dibromo-2,3-dimethylbutane in magic acid at −60 °C.