Hammett equation
[1][2] This equation was developed and published by Louis Plack Hammett in 1937[3] as a follow-up to qualitative observations in his 1935 publication.
[5] This notion does not follow from elemental thermochemistry or chemical kinetics and was introduced by Hammett intuitively.
for a given reaction rate with many differently substituted reactants will give a straight line.
These values, combined in the Hammett equation with K0 and remembering that ρ = 1, give the para substituent constants compiled in table 1 for amine, methoxy, ethoxy, dimethylamino, methyl, fluorine, bromine, chlorine, iodine, nitro and cyano substituents.
With meta substituents a carbon atom bearing the negative charge is further away from the carboxylic acid group (structure 2b).
This parameter is defined using the ionization constants of para substituted phenols, via a scaling factor to match up the values of σp– with those of σp for "non-anomalous" substituents, so as to maintain comparable ρ values: for ArOH ⇄ ArO– + H+, we define
Thus for reactions involving carbocations at the α-position, the σp values for electron-donating groups will appear insufficiently negative.
Based on similar considerations, a set of σp+ constants give better fit for reactions involving electron-donating groups at the para position and the formation of a carbocation at the benzylic site.
The σp+ are based on the rate constants of the SN1 reaction of cumyl chlorides in 90% acetone/water: for ArCMe2Cl + H2O → ArCMe2OH + HCl, we define
Note that the scaling factor is negative, since an electron-donating group speeds up the reaction.
As the value of ρ is related to the charge during the rate determining step, mechanisms can be devised based on this information.
If one of these mechanisms involves the formation of charge, this can be verified based on the ρ value.
For instance, a curve may show a sudden change in slope, or ρ value.
In such a situation, certain substituents may cause the transition state to appear earlier (or later) in the reaction mechanism.
Westheimer demonstrated that the electrical effects of π-substituted dipolar groups on the acidities of benzoic and phenylacetic acids can be quantitatively correlated, by assuming only direct electrostatic action of the substituent on the ionizable proton of the carboxyl group.
Such linear relationships correspond to linear free energy relationships, which strongly imply that the effect of the substituents are exerted through changes of potential energy and that the steric and entropy terms remain almost constant through the series.
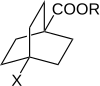
]octane-1-carboxylic acid derivatives, the substituent and reaction constants are designated σ’ and ρ’.
The plot of the Hammett equation is typically seen as being linear, with either a positive or negative slope correlating to the value of rho.
For the reason of the former case, new sigma constants have been introduced to accommodate the deviation from linearity otherwise seen resulting from the effect of the substituent.
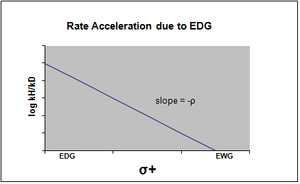
σ+ takes into account positive charge buildup occurring in the transition state of the reaction.
Therefore, an electron donating group (EDG) will accelerate the rate of the reaction by resonance stabilization and will give the following sigma plot with a negative rho value.
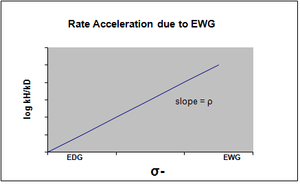
[12][non-primary source needed][non-primary source needed] σ- is designated in the case where negative charge buildup in the transition state occurs, and the rate of the reaction is consequently accelerated by electron withdrawing groups (EWG).
The EWG withdraws electron density by resonance and effectively stabilizes the negative charge that is generated.
This is attributed to the resonance contribution of the EWG to withdraw electron density thereby increasing the susceptibility for nucleophilic attack on the carbonyl carbon.
In fact, the sign of the charge and degree to which it develops will be affected by the substituent in the case of the benzylic system.
[vague][17][non-primary source needed][non-primary source needed] Core-electron binding energy (CEBE) shifts correlate linearly with the Hammett substituent constants (σ) in substituted benzene derivatives.
The image on the right shows four distinguished ring carbon atoms, C1(ipso), C2(ortho), C3(meta), C4(para) in p-F-C6H4-Z molecule.
1 is a product of a parameter κ and a Hammett substituent constant at the para position, σp.
ΔCEBEs of ring carbons in p-F-C6H4-Z were calculated with density functional theory to see how they correlate with Hammett σ-constants.
Hence the approximate agreement in numerical value and in sign between the CEBE shifts and their corresponding Hammett σ constant.