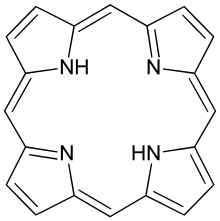
Porphyrin
Porphyrins (/ˈpɔːrfərɪns/ POR-fər-ins) are a group of heterocyclic, macrocyclic, organic compounds, composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges (=CH−).
In vertebrates, an essential member of the porphyrin group is heme, which is a component of hemoproteins, whose functions include carrying oxygen in the bloodstream.
In plants, an essential porphyrin derivative is chlorophyll, which is involved in light harvesting and electron transfer in photosynthesis.
[2][3] One result of the large conjugated system is that porphyrins typically absorb strongly in the visible region of the electromagnetic spectrum, i.e. they are deeply colored.
[9][10] Abelsonite is possibly the only geoporphyrin mineral, as it is rare for porphyrins to occur in isolation and form crystals.
Four PBGs are then combined through deamination into hydroxymethyl bilane (HMB), which is hydrolysed to form the circular tetrapyrrole uroporphyrinogen III.
The porphyria associated with the deficiency of each enzyme is also shown: A common synthesis for porphyrins is the Rothemund reaction, first reported in 1936,[12][13] which is also the basis for more recent methods described by Adler and Longo.
Porphyrins have been evaluated in the context of photodynamic therapy (PDT) since they strongly absorb light, which is then converted to heat in the illuminated areas.
[16] PDT is considered a noninvasive cancer treatment, involving the interaction between light of a determined frequency, a photo-sensitizer, and oxygen.
[17] These high reactive oxygen species react with susceptible cellular organic biomolecules such as; lipids, aromatic amino acids, and nucleic acid heterocyclic bases, to produce oxidative radicals that damage the cell, possibly inducing apoptosis or even necrosis.
[27] This isomer [18]porphyrin-(2.0.2.0) is named as porphycene, and the central N4 Cavity forms a rectangle shape as shown in figure.