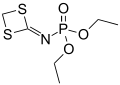
Phosphoramidate
In organophosphorus chemistry, phosphoramidates (sometimes also called amidophosphates) are a class of phosphorus compounds structurally related to phosphates (or organophosphates) via the substitution of an −O− group for an amine group (−N−).
The substitution of all three OH groups gives the phosphoric triamides (O=P(NR2)3), which are commonly referred to as phosphoramides.
[1] In the Stokes method, phosphoramidates are synthesized from phosphorus oxychloride.
The compound reacts with phenol to form a chlorophosphonate ester or diester, depending on stoichiometry.
The remaining chlorine substituents then react with an amine compound to give the phosphoramidate.