Aminophosphine
In organophosphorus chemistry, aminophosphines are compounds with the formula R3−nP(NR2)n where R is a hydrogen or organic substituent, and n = 0, 1, or 2.
Fundamental aminophosphines can not be isolated in a practical quantities but have been examined theoretically.
[5] Chlorodiphenylphosphine and diethylamine react to give an aminophosphine:[1][6] Primary amines react with phosphorus(III) chlorides to give aminophosphines with acidic α-NH centers:[7] Protic reagents attack the P-N bond.
Alcoholysis readily occurs: The P-N bond reverts to the chloride when treated with anhydrous hydrogen chloride: Transamination similarly converts one aminophosphine to another: With tris(dimethylamino)phosphine, dimethylamine evaporation can drive the equilibrium.
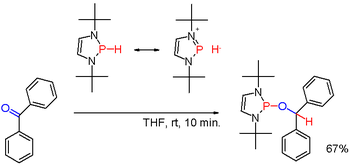
In diazaphospholenes the polarity of the P-H bond is inverted compared to traditional secondary phosphines.