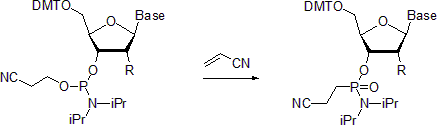
Nucleoside phosphoramidite
[1] To avoid undesired side reactions, reactive hydroxy and exocyclic amino groups present in natural or synthetic nucleosides are appropriately protected.
As long as a nucleoside analog contains at least one hydroxy group, the use of the appropriate protecting strategy allows one to convert that to the respective phosphoramidite and to incorporate the latter into synthetic nucleic acids.
Nucleoside phosphoramidites are relatively stable compounds with a prolonged shelf-life when stored as powders under anhydrous conditions in the absence of air at temperatures below 4 °C.
For instance, half-life of 2-cyanoethyl 5'-O-(4,4'-dimethoxytrityl)thymidine-3'-O-(N,N-diisopropylamino)phosphite in 95% aqueous acetonitrile at 25 °C is 200 h.[10] When water is served as a nucleophile, the product is an H-phosphonate diester as shown in Scheme above.
Due to the presence of residual water in solvents and reagents, the formation of the latter compound is the most common complication in the preparative use of phosphoramidites, particularly in oligonucleotide synthesis.
The naturally occurring nucleotides (nucleoside-3'- or 5'-phosphates) and their phosphodiester analogs are insufficiently reactive to afford an expeditious synthetic preparation of oligonucleotides in high yields.