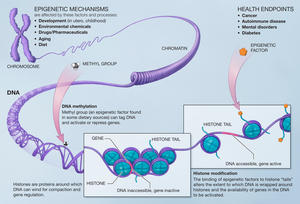
Histone methylation
Histone methylation, as a mechanism for modifying chromatin structure is associated with stimulation of neural pathways known to be important for formation of long-term memories and learning.
[4] This modification alters the properties of the nucleosome and affects its interactions with other proteins, particularly in regards to gene transcription processes.
The fundamental unit of chromatin, called a nucleosome, contains DNA wound around a protein octamer.
Lysine and arginine residues both contain amino groups, which confer basic and hydrophobic characteristics.
[9] Each addition of a methyl group on each residue requires a specific set of protein enzymes with various substrates and cofactors.
When dimethylated, the residue provides a platform for the binding of protein 53BP1 involved in the repair of double-stranded DNA breaks by non-homologous end joining.
In this way the integrity of the genome and epigenetic inheritance of genes are under the control of the actions of histone methyltransferases.
[15] However, because these processes are at times reversible, there is interest in utilizing their activities in concert with anti-cancer therapies.
Females, however, do not initially require both copies of the X chromosome as it would only double the amount of protein products transcribed as shown by the hypothesis of dosage compensation.
[16] This inactive X chromosome (Xi) is packed into an incredibly tight form of chromatin called heterochromatin.
[20] Through histone methylation, there is genetic imprinting, so that the same X homolog stays inactivated through chromosome replications and cell divisions.
It has been discovered that the deletion of genes that will eventually allow for the production of histone methyltransferase allows this organism to live as its lysine residues are not methylated.
[21] In recent years it has come to the attention of researchers that many types of cancer are caused largely due to epigenetic factors.
Since the discovery of oncogenes as well as tumor suppressor genes it has been known that a large factor of causing and repressing cancer is within our own genome.
If areas around oncogenes become unmethylated these cancer-causing genes have the potential to be transcribed at an alarming rate.
[23] In one-carbon metabolism, the amino acids glycine and serine are converted via the folate and methionine cycles to nucleotide precursors and SAM.
Multiple nutrients fuel one-carbon metabolism, including glucose, serine, glycine, and threonine.