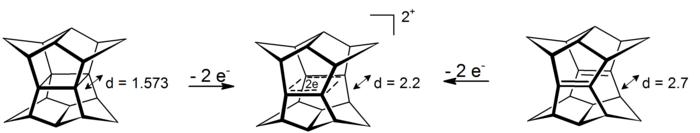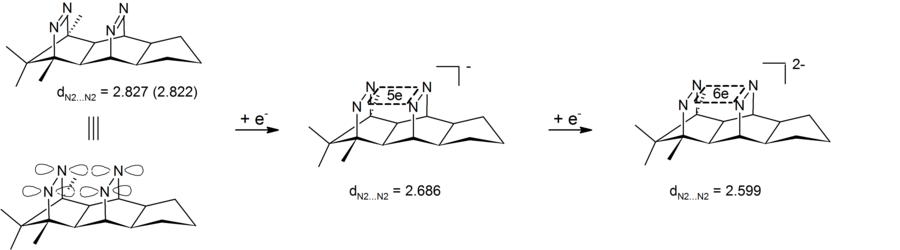Homoaromaticity
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom.
This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.
In a series of acetolysis experiments, Winstein et al. observed that the solvolysis reaction occurred empirically faster when the tosyl leaving group was in the equatorial position.
Classically, aromatic compounds were defined as planar molecules that possess a cyclically delocalized system of (4n+2)π electrons, satisfying Huckel's rule.
Most importantly, these conjugated ring systems are known to exhibit enormous thermochemical stability relative to predictions based on localized resonance structures.
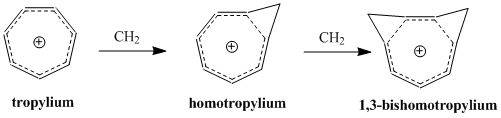
One of the best studied of these molecules is the homotropylium cation, the parent compound of which was first isolated as a stable salt by Pettit, et al. in 1962, when the group reacted cyclooctatraene with strong acids.
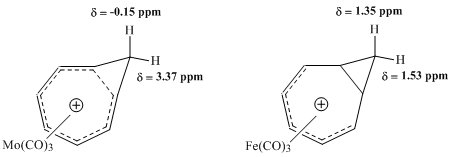
While characterizing the compound resulting from deprotonation of cyclooctatriene by 1H NMR spectroscopy, the group observed that the resonance corresponding to two protons bonded to the same methylene bridge carbon exhibited an astonishing degree of separation in chemical shift.
Instead, the group proposed the structure of the bicyclo[5.1.0]octadienyl compound, theorizing that the cyclopropane bond located on the interior of the eight-membered ring must be subject to considerable delocalization, thus explaining the dramatic difference in observed chemical shift.
Upon further consideration, Pettit was inclined to represent the compound as the "homotropylium ion," which shows the "internal cyclopropane" bond totally replaced by electron delocalization.
The magnetic field of the NMR could thus induce a ring current in the ion, responsible for the significant differences in resonance between the exo and endo protons of this methylene bridge.
Subsequent NMR studies undertaken by Winstein and others sought to evaluate the properties of metal carbonyl complexes with the homotropylium ion.
[9] An important piece of early evidence in support of the homotropylium cation structure that did not rely on the magnetic properties of the molecule involved the acquisition of its UV spectrum.
[6] More recently, work has been done to investigate the structure of the purportedly homoaromatic homotropylium ion by employing various other experimental techniques and theoretical calculations.
[6] The molecular orbital explanation of the stability of homoaromaticity has been widely discussed with numerous diverse theories, mostly focused on the homotropenylium cation as a reference.
[13] A significant second-order effect on the Perturbation Molecular Orbital model of homoaromaticity is the addition of a second homoconjugate linkage and its influence on stability.
In order to minimize δβ and thus keep the coupling term to a minimum, bishomoaromatic compounds form depending on the conformation of greatest stability by resonance and smallest steric hindrance.
The synthesis of the 1,3-bishomotropenylium cation by protonating cis-bicyclo[6.1.0]nona-2,4,6-triene agrees with theoretical calculations and maximizes stability by forming the two methylene bridges at the 1st and 3rd carbons.
If an inductively electron-donating group is attached to the cation at the 1st or 3rd carbon position, it has a stabilizing effect, improving the homoaromatic character of the compound.
In these 4-center-2-electron systems the delocalization happens in the plane that is defined by the four carbon atoms (prototype for the phenomenon of σ-aromaticity is cyclopropane which gains about 11.3 kcal mol−1 stability from the effect[16]).
UV and NMR analysis have shown that the aromatic character of this modified fulleroid is not disrupted by the addition of a homoconjugate linkage, therefore this compound is definitively homoaromatic.
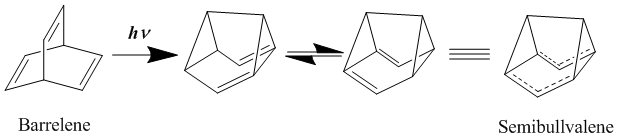
In an effort to further stabilize the delocalized transition structure by substituting semibullvalene with electron donating and accepting groups, it has been found that the activation barrier to this rearrangement can be lowered, but not eliminated.
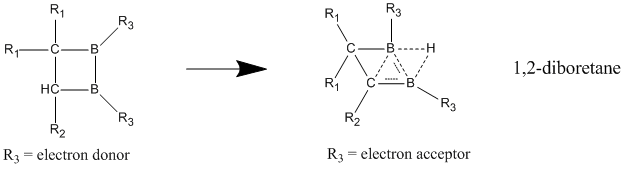
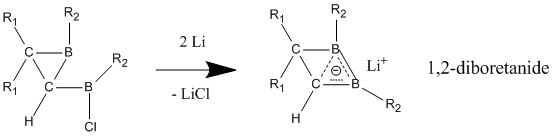
Homoaromatic character is best seen when electron-withdrawing groups are bonded to the boron atoms, causing the compound to adopt a nonclassical, delocalized structure.
Experiment results have shown the shortening of the transannular nitrogen-nitrogen distance, therefore demonstrating that dianionic bis-diazene is a type of anionic bishomoaromatic compound.