Hyaluronan synthase
[3] Two of the main differences between the isoforms are the chain length of the hyaluronan molecules that they produce and the ease with which they can be released from the cell surface.
[8] HAS-2 has also been proposed as a nanotherapeutic agent to combat osteoarthritis in joints displaying synovial inflammation as a result of increased hyaluronan depolymerization.
[10] HAS1 has a single catalytic domain and is embedded in the transmembrane channel to form glycosidic linkages in the production of hyaluronan (HA).
[12] Two catalytic residues exist in the catalytic domain; an aspartic acid to asparagine mutation at position 196 (D196N) leads to loss of GlcUA-transferase activity, and an aspartic acid to lysine mutation at position 477 (D477K) leads to loss of GlcNAc-transferase activity.
In COS-1 monkey kidney cells transfected with mouse HAS2 and HAS3 plasmids, one site of ubiquitination is seen on the lysine at residue 190.
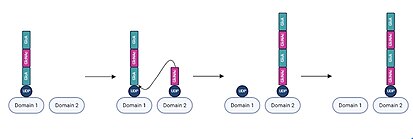
[11] The active site contains two distinct domains, each of which are capable of binding either the nascent UDP-hyaluronan chain or a UDP-sugar monomer.
[17] The process of monomer binding and elongation then repeats, with alternating GlcA and GlcNAc units being added as the UDP-hyaluronan chain shifts from one active site domain to the other.
[27] The HA produced by HAS also has been suggested to protect the cancer cell from physical damage while in the circulatory or lymphatic systems.
[28] The HA produced by HAS up-regulates secretion of various MMPs, proteolytic enzymes that are involved in many stages of the metastatic cascade.