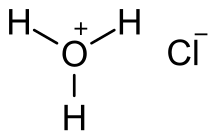
Hydrochloric acid
Both names are still used, especially in other languages, such as German: Salzsäure, Dutch: Zoutzuur, Afrikaans: Soutsuur, Swedish: Saltsyra, Finnish: Suolahappo, Spanish: Salfumán, Turkish: Tuz Ruhu, Polish: kwas solny, Hungarian: sósav, Czech: kyselina solná, Japanese: 塩酸 (ensan), Chinese: 盐酸 (yánsuān), and Korean: 염산 (yeomsan).
[15] Drawing on al-Razi's experiments, the De aluminibus et salibus ("On Alums and Salts"), an eleventh- or twelfth-century Arabic text falsely attributed to al-Razi and translated into Latin by Gerard of Cremona (1144–1187), described the heating of metals with various salts, which in the case of mercury resulted in the production of mercury(II) chloride (corrosive sublimate).
[16] In this process, hydrochloric acid actually started to form, but it immediately reacted with the mercury to produce corrosive sublimate.
Thirteenth-century Latin alchemists, for whom the De aluminibus et salibus was one of the main reference works, were fascinated by the chlorinating properties of corrosive sublimate, and they soon discovered that when the metals are eliminated from the process of heating vitriols, alums, and salts, strong mineral acids can directly be distilled.
This was first described in pseudo-Geber's De inventione veritatis ("On the Discovery of Truth", after c. 1300), where aqua regia was prepared by adding ammonium chloride to nitric acid.
The earliest recipes for the production of hydrochloric acid are found in Giovanni Battista Della Porta's (1535–1615) Magiae naturalis ("Natural Magic") and in the works of other contemporary chemists like Andreas Libavius (c. 1550–1616), Jean Beguin (1550–1620), and Oswald Croll (c. 1563–1609).
Italian chemist Ladislao Reti have summarized the result of their efforts thus:[22] The first clear instance of the preparation of hydrochloric acid appears in the writings of Della Porta, (1589 and 1608), Libavius (1597), pseudo-Basil (1604), van Helmont (1646) and Glauber (1648).
A new industrial process developed by Nicolas Leblanc of Issoudun, France enabled cheap large-scale production of sodium carbonate (soda ash).
In this Leblanc process, common salt is converted to soda ash, using sulfuric acid, limestone, and coal, releasing hydrogen chloride as a by-product.
An early exception was the Bonnington Chemical Works where, in 1830, the HCl began to be captured and the hydrochloric acid produced was used in making sal ammoniac (ammonium chloride).
Since hydrochloric acid was already fully settled as an important chemical in numerous applications, the commercial interest initiated other production methods, some of which are still used today.
After 2000, hydrochloric acid is mostly made by absorbing by-product hydrogen chloride from industrial organic compounds production.
[27] A combined IR, Raman, X-ray, and neutron diffraction study of concentrated hydrochloric acid showed that the hydronium ion forms hydrogen bonded complexes with other water molecules.
[5] A solution of hydrogen chloride in water behaves as a strong acid: the concentration of HCl molecules is effectively zero.
Physical properties of hydrochloric acid, such as boiling and melting points, density, and pH, depend on the concentration or molarity of HCl in the aqueous solution.
Higher concentrations up to just over 40% are chemically possible, but the evaporation rate is then so high that storage and handling require extra precautions, such as pressurization and cooling.
[25] In other countries, such as Italy, hydrochloric acid for domestic or industrial cleaning is sold as "Acido Muriatico", and its concentration ranges from 5% to 32%.
In less-demanding industry, technical quality hydrochloric acid suffices for neutralizing waste streams and swimming pool pH control.
Azeotropic, or "constant-boiling", hydrochloric acid (roughly 20.2%) can be used as a primary standard in quantitative analysis, although its exact concentration depends on the atmospheric pressure when it is prepared.
[34] Hydrochloric acid is used for a large number of small-scale applications, such as leather processing, household cleaning,[35] and building construction.
[25] Hydrochloric acid has been used for dissolving calcium carbonate, e.g. such things as de-scaling kettles and for cleaning mortar off brickwork.
[36] The stomach itself is protected from the strong acid by the secretion of a thick mucus layer, and by secretin induced buffering with sodium bicarbonate.
[8] Vapors or mists are a respiratory hazard, which can be partially mitigated by use of a respirator equipped with cartridges specifically designed to capture hydrochloric acid.
[citation needed] Hydrochloric acid has been listed as a Table II precursor under the 1988 United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances because of its use in the production of heroin, cocaine, and methamphetamine.