Hydrocyanation
The reaction involves the addition of hydrogen cyanide and requires a catalyst if the substrate alkene is unactivated.
Industrially, hydrocyanation is commonly performed on alkenes catalyzed by nickel complexes of phosphite (P(OR)3) ligands.
The intermediate M(H)(CN)Ln(alkene) then undergoes migratory insertion to give an alkylmetal cyanide.
The dicyanide arises via two pathways (L = phosphite):[1] Most alkenes are prochiral, and their hydrocyanation generates chiral nitriles.
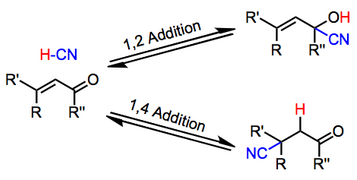
[5] Diastereoselectivity is generally high in these addition reactions, and the resulting β-cyano carbonyl compounds can be converted to a number of steroidal products.
The DuPont ADN process to give adiponitrile is shown below: This process consists of three steps: hydrocyanation of butadiene to a mixture of 2-methyl-butene-3-nitrile (2M3BM) and pentene-3-nitrile (3PN), an isomerization step from 2M3BM (not desired) to 3PN and a second hydrocyanation (aided by a Lewis acid cocatalyst such as aluminium trichloride or triphenylboron) to adiponitrile.
[8] Naproxen, an anti-inflammatory drug, is prepared via an asymmetric hydrocyanation of a vinylnaphthalene utilizing a phosphinite (OPR2) ligand, L .
[9] Some hydrocyanation catalysts generate a reversible equilibrium, and can transfer HCN units between two different alkenes.