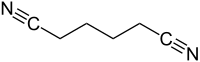
Adiponitrile
After patent application in 2004, the majority of adiponitrile is prepared by the nickel-catalysed hydrocyanation of butadiene, as discovered at DuPont, pioneered by William C. Drinkard.
In the final stage, these pentenenitriles are subjected to a second hydrocyanation to produce adiponitrile,[4] the anti-Markovnikov product, as well as 2-methylglutaronitrile, a useful byproduct.
Another side reaction is the alkene metathesis of 3-pentenenitrile to yield dicyanobutenes, which are readily hydrogenated to adiponitrile as described above.
[citation needed] The main producers of adiponitrile were:[8][9] BASF closed the 128 kt/y ADN plant at Seal Sands in 2009.
[12] The NIOSH recommended skin exposure limit for a work-related time weighted average concentration is 4ppm (18 mg/m3).