Hypervalent molecule
[3] Lewis maintained the importance of the two-center two-electron (2c-2e) bond in describing hypervalence, thus using expanded octets to account for such molecules.
[1] In 1990, Magnusson published a seminal work definitively excluding the significance of d-orbital hybridization in the bonding of hypervalent compounds of second-row elements.
[5] Nevertheless, a 2013 study showed that although the Pimentel ionic model best accounts for the bonding of hypervalent species, the energetic contribution of an expanded octet structure is also not null.
Consistent with this alternative view is the finding that hypercoordinated molecules based on fluorine ligands, for example PF5 do not have hydride counterparts, e.g. phosphorane (PH5) which is unknown.
On the other hand, some compounds that are normally written with ionic bonds in order to conform to the octet rule, such as ozone O3, nitrous oxide NNO, and trimethylamine N-oxide (CH3)3NO, are found to be genuinely hypervalent.
Early considerations of the geometry of hypervalent molecules returned familiar arrangements that were well explained by the VSEPR model for atomic bonding.
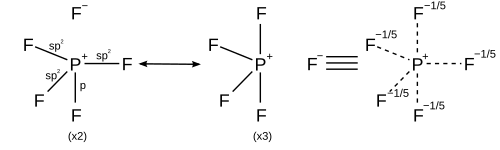
[5] It was shown that in the case of hexacoordinated SF6, d-orbitals are not involved in S-F bond formation, but charge transfer between the sulfur and fluorine atoms and the apposite resonance structures were able to account for the hypervalency (See below).
[3] A complete description of hypervalent molecules arises from consideration of molecular orbital theory through quantum mechanical methods.
An LCAO in, for example, sulfur hexafluoride, taking a basis set of the one sulfur 3s-orbital, the three sulfur 3p-orbitals, and six octahedral geometry symmetry-adapted linear combinations (SALCs) of fluorine orbitals, a total of ten molecular orbitals are obtained (four fully occupied bonding MOs of the lowest energy, two fully occupied intermediate energy non-bonding MOs and four vacant antibonding MOs with the highest energy) providing room for all 12 valence electrons.
Spin-coupled valence bond theory has been applied to diazomethane and the resulting orbital analysis was interpreted in terms of a chemical structure in which the central nitrogen has five covalent bonds; This led the authors to the interesting conclusion that "Contrary to what we were all taught as undergraduates, the nitrogen atom does indeed form five covalent linkages and the availability or otherwise of d-orbitals has nothing to do with this state of affairs.
This trend is also generally true of pentacoordinated main-group elements with one or more lone-pair-containing ligand, including the oxygen-pentacoordinated silicon examples shown below.
[28] Corriu and coworkers performed early work characterizing reactions thought to proceed through a hypervalent transition state.
This is followed by a nucleophilic attack of the intermediate by water in a rate determining step leading to hexacoordinated species that quickly decomposes giving the hydroxysilane.
Silane hydrolysis was further investigated by Holmes and coworkers [30] in which tetracoordinated Mes2SiF2 (Mes = mesityl) and pentacoordinated Mes2SiF−3 were reacted with two equivalents of water.
Additionally, X-ray diffraction data collected for the tetraethylammonium salts of the fluorosilanes showed the formation of hydrogen bisilonate lattice supporting a hexacoordinated intermediate from which HF−2 is quickly displaced leading to the hydroxylated product.
The Corriu group measured[31] Grignard reaction half-times by NMR for related 18-crown-6 potassium salts of a variety of tetra- and pentacoordinated fluorosilanes in the presence of catalytic amounts of nucleophile.
[32] Similar reactivity has also been observed for other hypervalent structures such as the miscellany of phosphorus compounds, for which hexacoordinated transition states have been proposed.
Alcoholysis of pentacoordinated phosphorus compounds, such as trimethoxyphospholene with benzyl alcohol, have also been postulated to occur through a similar octahedral transition state, as in hydrolysis, however without ring opening.
[34] It can be understood from these experiments that the increased reactivity observed for hypervalent molecules, contrasted with analogous nonhypervalent compounds, can be attributed to the congruence of these species to the hypercoordinated activated states normally formed during the course of the reaction.
Corriu and coworkers suggested that greater electropositive character at the pentavalent silicon atom may be responsible for its increased reactivity.
[36] A software program for ab initio calculations, Gaussian 86, was used by Dieters and coworkers to compare tetracoordinated silicon and phosphorus to their pentacoordinate analogues.
Advanced ab initio calculations were performed on series of tetracoordinated and pentacoordinated species to further understand this reactivity phenomenon.
[36] Addition of a fluoride ion to tetracoordinated silicon shows an overall average increase of 0.1 electron charge, which is considered insignificant.