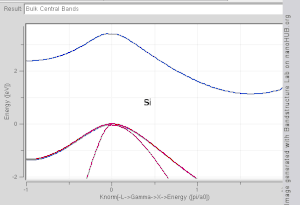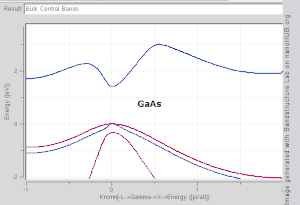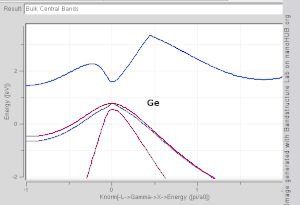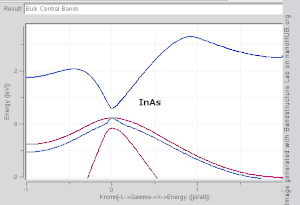
Direct and indirect band gaps
In an "indirect" gap, a photon cannot be emitted because the electron must pass through an intermediate state and transfer momentum to the crystal lattice.
For radiative recombination to occur in an indirect band gap material, the process must also involve the absorption or emission of a phonon, where the phonon momentum equals the difference between the electron and hole momentum.
The involvement of the phonon makes this process much less likely to occur in a given span of time, which is why radiative recombination is far slower in indirect band gap materials than direct band gap ones.
[clarification needed] At the edge of the loop, the planes above and beneath the "dislocation disk" are pulled apart, creating a negative pressure, which raises the energy of the conduction band substantially, with the result that the electrons cannot pass this edge.
[citation needed] The exact reverse of radiative recombination is light absorption.
Crystalline silicon is the most common solar-cell substrate material, despite the fact that it is indirect-gap and therefore does not absorb light very well.
As such, they are typically hundreds of microns thick; thinner wafers would allow much of the light (particularly in longer wavelengths) to simply pass through.
By comparison, thin-film solar cells are made of direct band gap materials (such as amorphous silicon, CdTe, CIGS or CZTS), which absorb the light in a much thinner region, and consequently can be made with a very thin active layer (often less than 1 micron thick).
The absorption spectrum of an indirect band gap material usually depends more on temperature than that of a direct material, because at low temperatures there are fewer phonons, and therefore it is less likely that a photon and phonon can be simultaneously absorbed to create an indirect transition.
For example, silicon is opaque to visible light at room temperature, but transparent to red light at liquid helium temperatures, because red photons can only be absorbed in an indirect transition.
[clarification needed] A common and simple method for determining whether a band gap is direct or indirect uses absorption spectroscopy.
By plotting certain powers of the absorption coefficient against photon energy, one can normally tell both what value the band gap is, and whether or not it is direct.
is related to light frequency according to the following formula:[1][2] where: This formula is valid only for light with photon energy larger, but not too much larger, than the band gap (more specifically, this formula assumes the bands are approximately parabolic), and ignores all other sources of absorption other than the band-to-band absorption in question, as well as the electrical attraction between the newly created electron and hole (see exciton).