Gallium arsenide
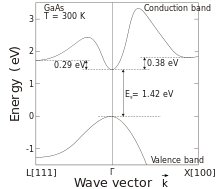
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure.
These superior properties are compelling reasons to use GaAs circuitry in mobile phones, satellite communications, microwave point-to-point links and higher frequency radar systems.
[citation needed] Another advantage of GaAs is that it has a direct band gap, which means that it can be used to absorb and emit light efficiently.
This has made it an ideal material for monolithic microwave integrated circuits (MMICs), where active and essential passive components can readily be produced on a single slice of GaAs.
One of the first GaAs microprocessors was developed in the early 1980s by the RCA Corporation and was considered for the Star Wars program of the United States Department of Defense.
[23] Other GaAs processors were implemented by the supercomputer vendors Cray Computer Corporation, Convex, and Alliant in an attempt to stay ahead of the ever-improving CMOS microprocessor.
Cray eventually built one GaAs-based machine in the early 1990s, the Cray-3, but the effort was not adequately capitalized, and the company filed for bankruptcy in 1995.
[citation needed] In addition, a Si crystal has a very stable structure and can be grown to very large diameter boules and processed with very good yields.
It is also a fairly good thermal conductor, thus enabling very dense packing of transistors that need to get rid of their heat of operation, all very desirable for design and manufacturing of very large ICs.
Naturally, a GaAs surface cannot withstand the high temperatures needed for diffusion; however a viable and actively pursued alternative as of the 1980s was ion implantation.
GaAs does not have a native oxide, does not easily support a stable adherent insulating layer, and does not possess the dielectric strength or surface passivating qualities of the Si-SiO2.
[28] Silicon has a nearly perfect lattice; impurity density is very low and allows very small structures to be built (down to 5 nm in commercial production as of 2020[29]).
[citation needed] Silicon has about three times the thermal conductivity of GaAs, with less risk of local overheating in high power devices.
[24] Gallium arsenide (GaAs) transistors are used in the RF power amplifiers for cell phones and wireless communicating.
[31] GaAs wafers are used in laser diodes, photodetectors, and radio frequency (RF) amplifiers for mobile phones and base stations.
[citation needed] In 1970, the GaAs heterostructure solar cells were developed by the team led by Zhores Alferov in the USSR,[37][38][39] achieving much higher efficiencies.
[42] GaAs-based photovoltaics are also responsible for the highest efficiency (as of 2022) of conversion of light to electricity, as researchers from the Fraunhofer Institute for Solar Energy Systems achieved a 68.9% efficiency when exposing a GaAs thin film photovoltaic cell to monochromatic laser light with a wavelength of 858 nanometers.
[44] In 2022, Rocket Lab unveiled a solar cell with 33.3% efficiency[45] based on inverted metamorphic multi-junction (IMM) technology.
A main advantage of the IMM process is that the inverted growth according to lattice mismatch allows a path to higher cell efficiency.
For example, GaAs-based photovoltaics show the best resistance to gamma radiation and high temperature fluctuations, which are of great importance for spacecraft.
However, other methods have been proposed that use phosphide-based materials and hydrochloric acid to achieve ELO with surface passivation and minimal post-etching residues and allows for direct reuse of the GaAs substrate.
At cryogenic temperatures it is among the brightest scintillators known[57][58][59] and is a promising candidate for detecting rare electronic excitations from interacting dark matter,[60] due to the following six essential factors: For this purpose an optical fiber tip of an optical fiber temperature sensor is equipped with a gallium arsenide crystal.
[70] The environment, health and safety aspects of gallium arsenide sources (such as trimethylgallium and arsine) and industrial hygiene monitoring studies of metalorganic precursors have been reported.