Intramolecular reaction
This configuration elevates the effective concentration of the reacting partners resulting in high reaction rates.
Intramolecular reactions, especially ones leading to the formation of 5- and 6-membered rings, are rapid compared to an analogous intermolecular process.
Finally, for 'large rings' (14-membered or higher), the rate constants level off, as the distance between the leaving group and nucleophile is now so large the reaction is now effectively intermolecular.
[1][2] Although the details may change somewhat, the general trends hold for a variety of intramolecular reactions, including radical-mediated and (in some cases) transition metal-catalyzed processes.
Longer tethers tend to generate the "straight" product where the terminal carbon of the alkene is linked to the
[8] Otherwise-intermolecular reactions can be made temporarily intramolecular by linking both reactants by a tether with all the advantages associated to it.
Popular choices of tether contain a carbonate ester, boronic ester, silyl ether, or a silyl acetal link (silicon tethers)[9][10] which are fairly inert in many organic reactions yet can be cleaved by specific reagents.
The main hurdle for this strategy to work is selecting the proper length for the tether and making sure reactive groups have an optimal orientation with respect to each other.


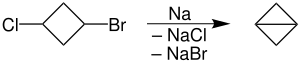

![Tethered intramolecular [2+2] reactions](http://upload.wikimedia.org/wikipedia/commons/8/88/23_fig._1.png)
![Effects of the length of tether on [2+2] photocyclization reaction](http://upload.wikimedia.org/wikipedia/commons/8/82/23_fig._2.png)
![Tethered [2+2] reaction in the total synthesis of (+) - Ginkgolide B](http://upload.wikimedia.org/wikipedia/commons/1/14/23_fig._3.png)