Intramolecular force
[2] The classical model identifies three main types of chemical bonds — ionic, covalent, and metallic — distinguished by the degree of charge separation between participating atoms.
[clarification needed] An ionic bond can be approximated as complete transfer of one or more valence electrons of atoms participating in bond formation, resulting in a positive ion and a negative ion bound together by electrostatic forces.
[5] This type of bond is generally formed between a metal and nonmetal, such as sodium and chlorine in NaCl.
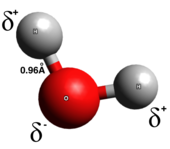
[6] Polar covalent bonds represent an intermediate type in which the electrons are neither completely transferred from one atom to another nor evenly shared.
Intramolecular forces are extremely important in the field of biochemistry, where it comes into play at the most basic levels of biological structures.
The main source of structure in these molecules is the interaction between the amino acid residues that form the foundation of proteins.