Inward-rectifier potassium channel
It is thought that this current may play an important role in regulating neuronal activity, by helping to stabilize the resting membrane potential of the cell.
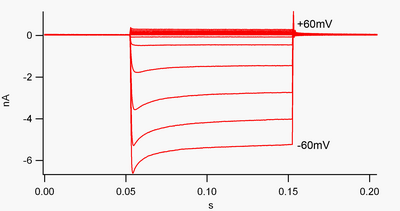
By convention, inward current (positive charge moving into the cell) is displayed in voltage clamp as a downward deflection, while an outward current (positive charge moving out of the cell) is shown as an upward deflection.
Simply put, this channel passes much more current in the inward direction than the outward one, at its operating voltage range.
These channels are not perfect rectifiers, as they can pass some outward current in the voltage range up to about 30 mV above resting potential.
[8] Inward rectifiers also differ from tandem pore domain potassium channels, which are largely responsible for "leak" K+ currents.
By mediating a small depolarizing K+ current at negative membrane potentials, they help establish resting membrane potential, and in the case of the Kir3 group, they help mediate inhibitory neurotransmitter responses, but their roles in cellular physiology vary across cell types: Voltage-dependence may be regulated by external K+, by internal Mg2+, by internal ATP and/or by G-proteins.
Inward rectifiers lack the intrinsic voltage sensing helices found in many VIC family channels.
In a few cases, those of Kir1.1a, Kir6.1 and Kir6.2, for example, direct interaction with a member of the ABC superfamily has been proposed to confer unique functional and regulatory properties to the heteromeric complex, including sensitivity to ATP.
It transports monovalent cations with the selectivity: K ≈ Rb ≈ Cs ≫ Li ≈ Na ≈ NMGM (protonated N-methyl-D-glucamine).