Electrochemical gradient
It appears in electroanalytical chemistry and has industrial applications such as batteries and fuel cells.
In biology, electrochemical gradients allow cells to control the direction ions move across membranes.
The combination of these two phenomena determines the thermodynamically-preferred direction for an ion's movement across the membrane.
[2]: 403 [3] The combined effect can be quantified as a gradient in the thermodynamic electrochemical potential:[citation needed]
In a battery, an electrochemical potential arising from the movement of ions balances the reaction energy of the electrodes.
[citation needed] The generation of a transmembrane electrical potential through ion movement across a cell membrane drives biological processes like nerve conduction, muscle contraction, hormone secretion, and sensation.
By convention, physiological voltages are measured relative to the extracellular region; a typical animal cell has an internal electrical potential of (−70)–(−50) mV.
The final step of cellular respiration is the electron transport chain, composed of four complexes embedded in the inner mitochondrial membrane.
Complexes I, III, and IV pump protons from the matrix to the intermembrane space (IMS); for every electron pair entering the chain, ten protons translocate into the IMS.
The energy resulting from the flux of protons back into the matrix is used by ATP synthase to combine inorganic phosphate and ADP.
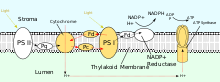
[6][2]: 743–745 Similar to the electron transport chain, the light-dependent reactions of photosynthesis pump protons into the thylakoid lumen of chloroplasts to drive the synthesis of ATP.
Of the proteins that participate in noncyclic photophosphorylation, photosystem II (PSII), plastiquinone, and cytochrome b6f complex directly contribute to generating the proton gradient.
[2]: 769–770 Several other transporters and ion channels play a role in generating a proton electrochemical gradient.
One is TPK3, a potassium channel that is activated by Ca2+ and conducts K+ from the thylakoid lumen to the stroma, which helps establish the electric field.
On the other hand, the electro-neutral K+ efflux antiporter (KEA3) transports K+ into the thylakoid lumen and H+ into the stroma, which helps establish the pH gradient.
This makes the inside of the cell more negative than the outside and more specifically generates a membrane potential Vmembrane of about −60 mV.
Formally, the molar Gibbs free energy change associated with successful transport is[citation needed]
[citation needed] Proton gradients in particular are important in many types of cells as a form of energy storage.
The gradient is usually used to drive ATP synthase, flagellar rotation, or metabolite transport.
[15] This section will focus on three processes that help establish proton gradients in their respective cells: bacteriorhodopsin and noncyclic photophosphorylation and oxidative phosphorylation.
In bacteriorhodopsin, the proton pump is activated by absorption of photons of 568nm wavelength, which leads to isomerization of the Schiff base (SB) in retinal forming the K state.
The protein then shifts to the M2 state by separating Glu204 from Glu194 which releases a proton from Glu204 into the external medium.
Absorption of photons of 680nm wavelength is used to excite two electrons in P680 to a higher energy level.
This reduces plastoquinone (PQ) to plastoquinol (PQH2) which is released from PSII after gaining two protons from the stroma.
After being released from PSII, PQH2 travels to the cytochrome b6f complex, which then transfers two electrons from PQH2 to plastocyanin in two separate reactions.
The process that occurs is similar to the Q-cycle in Complex III of the electron transport chain.
[2]: 782–783 [17] In the electron transport chain, complex I (CI) catalyzes the reduction of ubiquinone (UQ) to ubiquinol (UQH2) by the transfer of two electrons from reduced nicotinamide adenine dinucleotide (NADH) which translocates four protons from the mitochondrial matrix to the IMS:[18]
The first step involving the transfer of two electrons from the UQH2 reduced by CI to two molecules of oxidized cytochrome c at the Qo site.
Complex IV (CIV) catalyzes the transfer of two electrons from the cytochrome c reduced by CIII to one half of a full oxygen.
Utilizing one full oxygen in oxidative phosphorylation requires the transfer of four electrons.