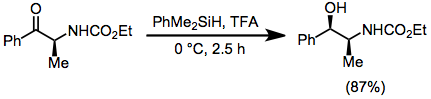Ionic hydrogenation
Many alcohols, alkyl halides, acetals, orthoesters, alkenes, aldehydes, ketones, and carboxylic acid derivatives are suitable substrates.
Only reactive electrophiles undergo reduction, selectivity is possible in reactions of substrates with multiple reducible functional groups.
Upon the generation of a carbocation, rate-determining hydride transfer from the organosilane occurs to yield a reduced product.
Retention of configuration at silicon has been observed in silane reductions of chiral triaryl methyl chlorides in benzene.
[4] Polymeric hydrosilanes, such as polymethylhydrosiloxane (PHMS) may be employed to facilitate separation of the reduced products from silicon-containing byproducts.
In the case of metal-catalyzed ionic hydrogenation, the substrates and their products must not bind to metal sites, as this would interfere with H2 activation.
TH catalysts however do not employ strong acids and both the H− and H+ components are covalently bonded to the complex prior to transfer to the unsaturated substrates.