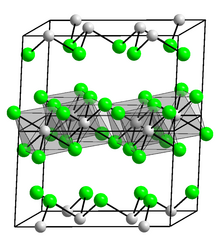
Iridium(III) iodide
Iridium(III) iodide can be obtained by reducing iridium(IV) iodide with hydrogen at 210 °C.
[2] It can also be formed by the reaction of iridium dioxide[3] or iridium(III) hydroxide with hydrogen iodide.
[4] Iridium(III) iodide is a dark brown solid that is insoluble in water.
[5][6] Its trihydrate is yellow and can be dehydrated into the dihydrate or anhydrous form on heating.
Iridium(III) iodide also has a monohydrate.