Isotachophoresis
It is a form of electrophoresis; charged analytes are separated based on ionic mobility, a quantity which tells how fast an ion migrates through an electric field.
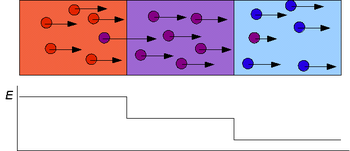
If present in sufficient amounts, focusing analyte ions can displace all electrolyte co-ions, reaching a plateau concentration.
[1] A completed ITP separation is characterized by a dynamic equilibrium in which all coionic zones migrate with equal velocities.
From this phenomenon ITP has obtained its name: iso = equal, tachos = speed, phoresis = migration.
When all of the TE ions are dissolved, the focusing process ceases and the analytes are separated according to the principles of zone electrophoresis.

White: leading electrolyte; gray: terminating electrolyte; hatched: the analytes