Isothermal process
Isothermal processes can occur in any kind of system that has some means of regulating the temperature, including highly structured machines, and even living cells.
[1] In the thermodynamic analysis of chemical reactions, it is usual to first analyze what happens under isothermal conditions and then consider the effect of temperature.
[2] Phase changes, such as melting or evaporation, are also isothermal processes when, as is usually the case, they occur at constant pressure.
This is a consequence of Joule's second law which states that the internal energy of a fixed amount of an ideal gas depends only on its temperature.
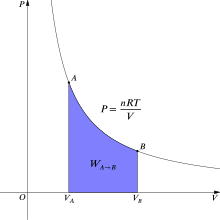
[5] In the isothermal compression of a gas there is work done on the system to decrease the volume and increase the pressure.
To maintain the constant temperature energy must leave the system as heat and enter the environment.
In either case, with the aid of a suitable linkage the change in gas volume can perform useful mechanical work.
Since the first law of thermodynamics states that ΔU = Q + W in IUPAC convention, it follows that Q = −W for the isothermal compression or expansion of ideal gases.
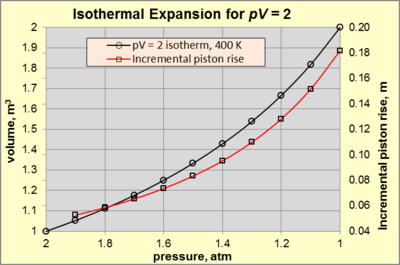
Consider a working gas in a cylindrical chamber 1 m high and 1 m2 area (so 1m3 volume) at 400 K in static equilibrium.
Isothermal expansion continues as long as the applied force decreases and appropriate heat is added to keep pV = 2 [atm·m3] (= 2 atm × 1 m3).
This is the maximum amount of usable mechanical work obtainable from the process at the stated conditions.
For example, a pressure decrease from 2 to 1.9 atm causes a piston rise of 0.0526 m. In comparison, a pressure decrease from 1.1 to 1 atm causes a piston rise of 0.1818 m. Isothermal processes are especially convenient for calculating changes in entropy since, in this case, the formula for the entropy change, ΔS, is simply where Qrev is the heat transferred (internally reversible) to the system and T is absolute temperature.
A simple example is an equilibrium phase transition (such as melting or evaporation) taking place at constant temperature and pressure.
If the transition takes place under such equilibrium conditions, the formula above may be used to directly calculate the entropy change[7] Another example is the reversible isothermal expansion (or compression) of an ideal gas from an initial volume VA and pressure PA to a final volume VB and pressure PB.
Note that the result Q = 0 for the free expansion can not be used in the formula for the entropy change since the process is not reversible.