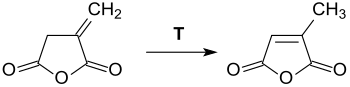
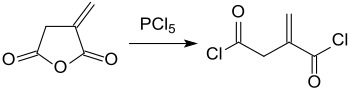
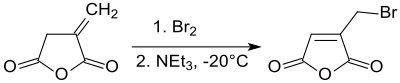
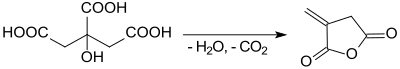Itaconic anhydride
[8] According to an organic synthesis protocol,[4] itaconic anhydride is obtained from the rapid heating of citric acid monohydrate in a modest yield (37-47 %).
[9] Also when heating anhydrous citric acid to 260 °C in a vacuum, a mixture of itaconic and citraconic anhydride is achieved "in good yield".
[19] Otto Diels and Kurt Alder already described the addition (Diels-Alder reaction) of the dienophile itaconic anhydride to the diene cyclopentadiene in 1928.
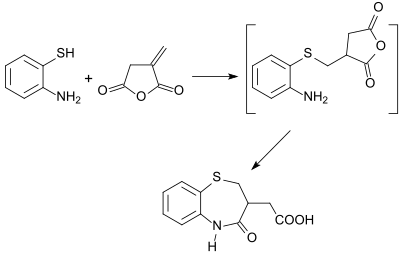
With other nucleophiles, such as alcohols, ammonia,[23] amines and hydroxylamine, itaconic anhydride reacts regioselectively in position 3 to the corresponding esters, amides and hydroxamic acids.
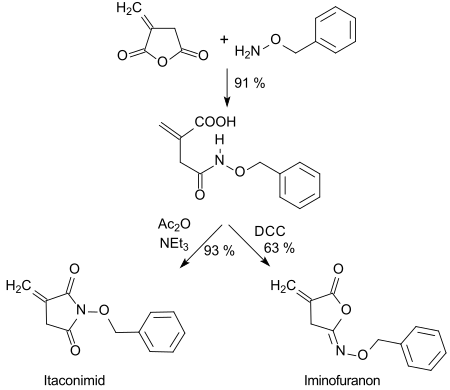
The hydroxamic acid formed with O-benzylhydroxylamine can be cyclized in high yields with dicyclohexylcarbodiimide (DCC) to five-membered isoimides (iminofuranones) or with acetanhydride (Ac2O) to imides.
[30] Functional polymers exclusively from biogenic monomers involves the ring-opening metathesis polymerisation of an oxanorbornene ester produced from itaconic anhydride and furfuryl alcohol by Diels-Alder lactonisation using a Grubbs II catalyst.