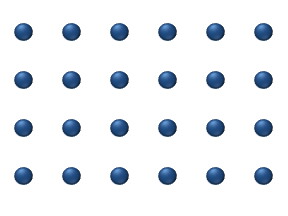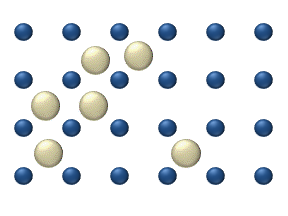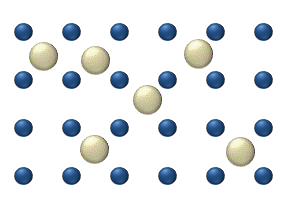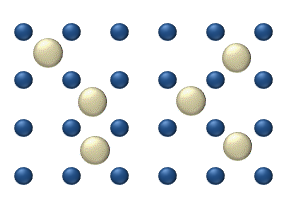Lattice diffusion coefficient
In condensed matter physics, lattice diffusion (also called bulk or volume diffusion) refers to atomic diffusion within a crystalline lattice,[1] which occurs by either interstitial or substitutional mechanisms.
In interstitial lattice diffusion, a diffusant (such as carbon in an iron alloy), will diffuse in between the lattice structure of another crystalline element.
In substitutional lattice diffusion (self-diffusion for example), the atom can only move by switching places with another atom.
Substitutional lattice diffusion is often contingent upon the availability of point vacancies throughout the crystal lattice.
Diffusing particles migrate from point vacancy to point vacancy by the rapid, essentially random jumping about (jump diffusion).
Since the prevalence of point vacancies increases in accordance with the Arrhenius equation, the rate of crystal solid state diffusion increases with temperature.
For a single atom in a defect-free crystal, the movement can be described by the "random walk" model.
The movement of atoms can be described as jumps, and the interstitial diffusion coefficient depends on the jump frequency.
can be expressed as the sum of activation enthalpy term
where The diffusion coefficient can be simplified to an Arrhenius equation form:
where In the case of interstitial diffusion, the activation enthalpy
is only dependent on the activation energy barrier to the movement of interstitial atoms from one site to another.
The diffusion coefficient increases exponentially with temperature at a rate determined by the activation enthalpy
The rate of self-diffusion can be measured experimentally by introducing radioactive A atoms (A*) into pure A and measuring the rate at which penetration occurs at various temperatures.
The diffusion coefficient of A* and A can be related to the jump frequency and expressed as:
An atom can make a successful jump when there are vacancies nearby and when it has enough thermal energy to overcome the energy barrier to migration.
The number of successful jumps an atom will make in one second, or the jump frequency, can be expressed as:
The diffusion coefficient in thermodynamic equilibrium can be expressed with
Substituting ΔG = ΔH – TΔS gives:
The diffusion coefficient can be simplified to an Arrhenius equation form:
where Compared to that of interstitial diffusion, the activation energy for self-diffusion has an extra term (ΔHv).
Since self-diffusion requires the presence of vacancies whose concentration depends on ΔHv.
It is the same process as the jumping of an atom into a vacant site but without the need to consider the probability of vacancy presence, since a vacancy is usually always surrounded by atom sites to which it can jump.
A vacancy can have its own diffusion coefficient that is expressed as:
The diffusion coefficient can also be expressed in terms of enthalpy of migration (
) of a vacancy, which are the same as for the migration of a substitutional atom:
In a system with multiple components (e.g. a binary alloy), the solvent (A) and the solute atoms (B) will not move in an equal rate.
Each atomic species can be given its own intrinsic diffusion coefficient
, expressing the diffusion of a certain species in the whole system.
are the amount fractions of species A and B, respectively.