X-ray diffraction
In many cases these diffraction patterns can be Interpreted using a single scattering or kinematical theory with conservation of energy (wave vector).
Barkla created the x-ray notation for sharp spectral lines, noting in 1909 two separate energies, at first, naming them "A" and "B" and, supposing that there may be lines prior to "A", he started an alphabet numbering beginning with "K."[2][3] Single-slit experiments in the laboratory of Arnold Sommerfeld suggested that X-rays had a wavelength of about 1 angstrom.
[4] X-rays are not only waves but also have particle properties causing Sommerfeld to coin the name Bremsstrahlung for the continuous spectra when they were formed when electrons bombarded a material.
[3] Albert Einstein introduced the photon concept in 1905,[5] but it was not broadly accepted until 1922,[6][7] when Arthur Compton confirmed it by the scattering of X-rays from electrons.
The idea that crystals could be used as a diffraction grating for X-rays arose in 1912 in a conversation between Paul Peter Ewald and Max von Laue in the English Garden in Munich.
Von Laue worked with two technicians, Walter Friedrich and his assistant Paul Knipping, to shine a beam of X-rays through a copper sulfate crystal and record its diffraction pattern on a photographic plate.
The results were presented to the Bavarian Academy of Sciences and Humanities in June 1912 as "Interferenz-Erscheinungen bei Röntgenstrahlen" (Interference phenomena in X-rays).
[15][16] After seeing the initial results, Laue was walking home and suddenly conceived of the physical laws describing the effect.
[14]: 44 Laue developed a law that connects the scattering angles and the size and orientation of the unit-cell spacings in the crystal, for which he was awarded the Nobel Prize in Physics in 1914.
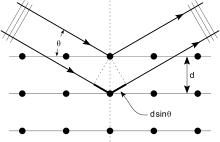
A reflection is said to be indexed when its Miller indices (or, more correctly, its reciprocal lattice vector components) have been identified from the known wavelength and the scattering angle 2θ.
[21] Each X-ray diffraction pattern represents a spherical slice of reciprocal space, as may be seen by the Ewald sphere construction.
Consider the fraction of scattered waves that leave with an outgoing wave-vector of kout and strike a screen (detector) at rscreen.
From the time that the photon is scattered at r until it is absorbed at rscreen, the photon undergoes a change in phase The net radiation arriving at rscreen is the sum of all the scattered waves throughout the crystal which may be written as a Fourier transform where g = kout – kin is a reciprocal lattice vector that satisfies Bragg's law and the Ewald construction mentioned above.
[23][24] Small scale diffraction experiments can be done with a local X-ray tube source, typically coupled with an image plate detector.
The most common metal used is copper, which can be kept cool easily due to its high thermal conductivity, and which produces strong Kα and Kβ lines.
X-ray beams are generated in synchrotrons which accelerate electrically charged particles, often electrons, to nearly the speed of light and confine them in a (roughly) circular loop using magnetic fields.
Cryo crystallography can protect the sample from radiation damage, by freezing the crystal at liquid nitrogen temperatures (~100 K).
As each crystal is randomly oriented in the beam, hundreds of thousands of individual diffraction images must be collected in order to get a complete data set.
In general, single-crystal X-ray diffraction offers more structural information than these other techniques; however, it requires a sufficiently large and regular crystal, which is not always available.
The Laue back reflection mode records X-rays scattered backwards from a broad spectrum source.
Hence electron beams produce strong multiple or dynamical scattering even for relatively thin crystals (>10 nm).
Therefore, neutron scattering is useful for observing the positions of light atoms with few electrons, especially hydrogen, which is essentially invisible in X-ray diffraction.