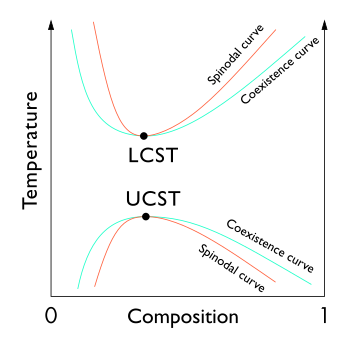Lower critical solution temperature
The phase behavior of polymer solutions is an important property involved in the development and design of most polymer-related processes.
[3][4] In the phase diagram of the mixture components, the LCST is the shared minimum of the concave up spinodal and binodal (or coexistence) curves.
[1][2] The nicotine-water system has an LCST of 61 °C, and also a UCST of 210 °C at pressures high enough for liquid water to exist at that temperature.
[18] For nonpolar systems such as polystyrene in cyclohexane, phase separation has been observed in sealed tubes (at high pressure) at temperatures approaching the liquid-vapor critical point of the solvent.
These methods require experimental data to adjust the unknown parameters, resulting in limited predictive ability .
[21][22] A new approach proposed by Liu and Zhong develops linear models for the prediction of θ(LCST) using molecular connectivity indices, which depends only on the solvent and polymer structures.
QSAR/QSPR studies constitute an attempt to reduce the trial-and-error element in the design of compounds with desired activity/properties by establishing mathematical relationships between the activity/property of interest and measurable or computable parameters, such as topological, physicochemical, stereochemistry, or electronic indices.
[25] Using validated robust QSPR models, experimental time and effort can be reduced significantly as reliable estimates of θ (LCST) for polymer solutions can be obtained before they are actually synthesized in the laboratory.