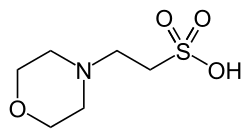
MES (buffer)
MES (2-(N-morpholino)ethanesulfonic acid) is a chemical compound that contains a morpholine ring.
MOPS is a similar pH buffering compound which contains a propanesulfonic moiety instead of an ethanesulfonic one.
The pH (and pKa at ionic strength I≠0) of the buffer solution changes with concentration and temperature, and this effect may be predicted using online calculators.
These buffers were developed with the following criteria in mind: midrange pKa, maximum water solubility and minimum solubility in all other solvents, minimal salt effects, minimal change in pKa with temperature, chemically and enzymatically stable, minimal absorption in visible or UV spectral range and reasonably easily synthesized.
[1][3] Commercial preparations of MES (and other sulfonylethyl buffers like BES, CHES, and PIPES) can contain a contaminant oligo(vinylsulfonic acid) (OVS), which is a polyanionic mimic of RNA, and can be a potent (pM) inhibitor of RNA binding proteins and enzymes.