Maltase
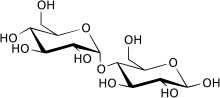
Maltase is an informal name for a family of enzymes that catalyze the hydrolysis of disaccharide maltose into two simple sugars of glucose.
[1][2] Maltases are members of a group of intestinal enzymes called FamilyGH13 (Glycoside hydrolase family 13) that are responsible for breaking apart the α-glucosidase linkages of complex carbohydrates into simple to use glucose molecules.
[4] Other than brewing, maltose glucoamylase has been studied by introducing specific inhibitors to stop the hydrolysis of the α-glucosidase linkages.
In 1833 French chemists Anselm Payen and Jean-Francois Persoz discovered a malt extract that converted starch into glucose which they called diastase at the time.
[2] In the 1960s advances in protein chemistry allowed Arne Dahlqvist and Giorgio Semenza to fractionate and characterize small intestinal maltase activities.
Both groups showed there were four major fractions of maltase activity that were intrinsic to two different peptide structures, sucrase-isomaltase and maltase-glucoamylase.
[1][2][9][6] Fifty years later entering the genomic age, cloning and sequencing of the mucosal starch hydrolase confirmed Dahlqvist and Semenza's findings.
[10][11] AMD is a non sex linked autosomal recessive condition in which excessive accumulation of glycogen build up within lysosome vacuoles in nearly all types of cells all over the body.