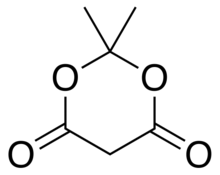
Meldrum's acid
The resulting anion [C6H7O4]− is stabilized by resonance between the two alternatives, so that the double bond is delocalized and each oxygen in the carbonyls has a formal charge of −1/2.
However, while dimedone exists in solution predominantly as the mono-enol tautomer, Meldrum's acid is almost entirely as the diketone form.
In 2004, Ohwada and coworkers determined that the energy-minimizing conformation structure of the compound places the alpha proton's σ*CH orbital in the proper geometry to align with the π*CO, so that the ground state poses unusually strong destabilization of the C-H bond.
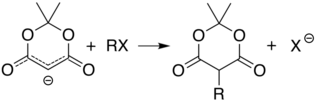
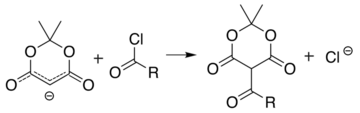
[6][7][8][9] Ketoesters formed from the reaction of alcohols with Meldrum's acid derivatives are useful in the Knorr pyrrole synthesis.
Alternately, the pyrolysis can be performed in solution, to obtain the same results without isolating the ketene, in a one-pot reaction.
The ability to form such diverse products makes Meldrum's acid a very useful reagent for synthetic chemists.