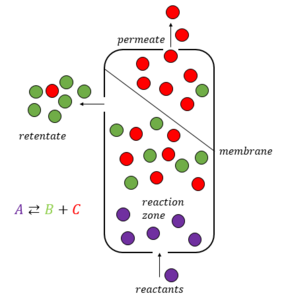
Membrane reactor
[3] The integration of reaction section with selective extraction of a reactant allows an enhancement of the conversions compared to the equilibrium value.
[5] If temperature and pressure are fixed, this equilibrium state is a constraint for the ratio of products versus reactants concentrations, obstructing the possibility to reach higher conversions.
[9] Today hydrogen is mainly used in chemical industry as a reactant in ammonia production and methanol synthesis, and in refinery processes for hydrocracking.
[10] More than 50% of hydrogen is currently produced from steam reforming of natural gas, due to low costs and the fact that it is a mature technology.
[11] Traditional processes are composed by a steam reforming section, to produce syngas from natural gas, two water gas shift reactors which enhance hydrogen in syngas and a pressure swing adsorption unit for hydrogen purification.
[12] Membrane reactors make a process intensification including all these sections in one single unit, with both economic and environmental benefits.
[14] Among membranes, dense inorganic are the most suitable having a selectivity orders of magnitude bigger than porous ones.
[citation needed] The production of chloride (Cl2) and caustic soda NaOH from NaCl is carried out industrially by the chlor-alkali-process using a proton conducting polyelectrolyte membrane.
[18] The advantage of this method over other forms of immobilization of the catalyst is that the enzymes are not altered in activity or selectivity as it remains solubilized.
[citation needed] The principle can be applied to all macromolecular catalysts which can be separated from the other reactants by means of filtration.
In the STAR process[citation needed] for the catalytic conversion of methane from natural gas with oxygen from air, to methanol by the partial oxidation 2CH4 + O2