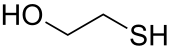
2-Mercaptoethanol
ME or βME, as it is commonly abbreviated, is used to reduce disulfide bonds and can act as a biological antioxidant by scavenging hydroxyl radicals (amongst others).
Due to its diminished vapor pressure, its odor, while unpleasant, is less objectionable than related thiols.
[5] Some proteins can be denatured by 2-mercaptoethanol, which cleaves the disulfide bonds that may form between thiol groups of cysteine residues.
[8][9] 2-Mercaptoethanol and related reducing agents (e.g., DTT) are often included in enzymatic reactions to inhibit the oxidation of free sulfhydryl residues, and hence maintain protein activity.
[11] Some carbamate protecting groups such as carboxybenzyl (Cbz) or allyloxycarbonyl (alloc) can be deprotected using 2-mercaptoethanol in the presence of potassium phosphate in dimethylacetamide.