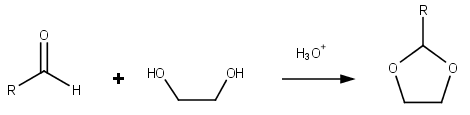
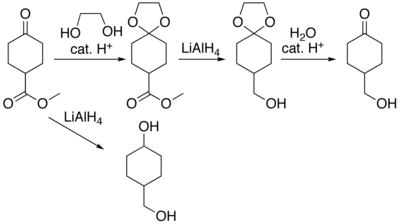
Dioxolane
Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol.
[8] Neosporol is a natural product that includes a 1,3-dioxolane moiety, and is an isomer of sporol which has a 1,3-dioxane ring.
[9] The total synthesis of both compounds has been reported, and each includes a step in which a dioxolane system is formed using trifluoroperacetic acid (TFPAA), prepared by the hydrogen peroxide – urea method.
[10] In the case of neosporol, a Prilezhaev reaction[13] with trifluoroperacetic acid is used to convert a suitable allyl alcohol precursor to an epoxide, which then undergoes a ring-expansion reaction with a proximate carbonyl functional group to form the dioxolane ring.
[10][11] A similar approach is used in the total synthesis of sporol, with the dioxolane ring later expanded to a dioxane system.