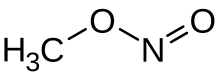
Methyl nitrite
At room temperature, methyl nitrite exists as a mixture of cis and trans conformers.
[5] Note that nitrogen is a better nucleophile than oxygen and most nitrites would react via an SN2-like mechanism and the major product would be nitromethane.
However, the presence of the silver ion in solution has a stabilizing effect on the formation of carbocation intermediates, increasing the percent yield of methyl nitrite.
[4] The figure shows the two gas-phase structures of methyl nitrite, as determined by IR and microwave spectroscopy.
[7] As one product of the combustion of unleaded petrol in air, methyl nitrite has been proposed as a cause of the decline of insects, and hence that of songbirds in Europe.