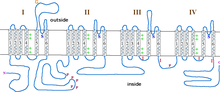
L-type calcium channel
L-type calcium channels are responsible for the excitation-contraction coupling of skeletal, smooth, cardiac muscle, and for aldosterone secretion in endocrine cells of the adrenal cortex.
[1] They are also found in neurons, and with the help of L-type calcium channels in endocrine cells, they regulate neurohormones and neurotransmitters.
They have also been seen to play a role in gene expression, mRNA stability, neuronal survival, ischemic-induced axonal injury, synaptic efficacy, and both activation and deactivation of other ion channels.
[5] In skeletal muscle, there is a very high concentration of L-type calcium channels, situated in the T-tubules.
In 1953, Paul Fatt and Bernard Katz discovered voltage gated calcium channels in crustacean muscle.
[11] As the pore opens and causes an influx of calcium, calcium binds to calmodulin and then interacts with the loop that connects the adjacent EF-hand motifs and causes a conformational change in the EF-hand motif so it interacts with the pore to cause quick inhibition in the channel.
Hydrophobic pockets in the Ca2+/Cam complex will also bind to three sections of the IQ domain known as the “aromatic anchors”.
One of the most recognized characteristics of the L-type calcium channel is its unique sensitivity to 1,4-dihydropyridines (DHPs).
The two helices can form a structure that bind competitively with CaM to reduce the open-state probability and lower calcium-dependent inhibition (CDI).
[6] L-type calcium channels are also modulated by G protein-coupled receptors and the adrenergic nervous system.
Activated Phospholipase C (PLC) from G protein-coupled receptors can breakdown polyphosphoinositides to decrease the channel's calcium current by 20%-30%.
[6]This article incorporates text from the United States National Library of Medicine, which is in the public domain.