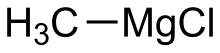Methylmagnesium chloride
This highly flammable, colorless, and moisture sensitive material is the simplest Grignard reagent and is commercially available, usually as a solution in tetrahydrofuran.
It is prepared by the reaction of methyl chloride and magnesium in ethyl ether.
[2] As with most Grignard reagents, methylmagnesium chloride is highly solvated by ether solvents via coordination from two oxygen atoms to give a tetrahedrally bonded magnesium center.
It reacts with water and other protic reagents to give methane, e.g.,: When treated with dioxane, ether solutions of methylmagnesium chloride reacts to give the insoluble coordination polymer with the formula MgCl2(dioxane)2.
This conversion exploits the Schlenk equilibrium, which is driven to the right by the precipitation of the magnesium halide:[3]