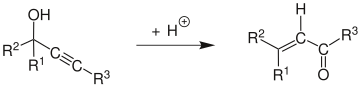
Meyer–Schuster rearrangement
[1] Reviews have been published by Swaminathan and Narayan,[2] Vartanyan and Banbanyan,[3] and Engel and Dudley,[4] the last of which describes ways to promote the Meyer–Schuster rearrangement over other reactions available to propargyl alcohols.
Attack of a water molecule on the carbocation and deprotonation is followed by tautomerization to give the α,β-unsaturated carbonyl compound.
[8] The reaction of tertiary alcohols containing an α-acetylenic group does not produce the expected aldehydes, but rather α,β-unsaturated methyl ketones via an enyne intermediate.
While the traditional Meyer–Schuster rearrangement uses harsh conditions with a strong acid as the catalyst, this introduces competition with the Rupe reaction if the alcohol is tertiary.
[13] An example from their paper is given below: The Meyer–Schuster rearrangement has been used in a variety of applications, from the conversion of ω-alkynyl-ω-carbinol lactams into enamides using catalytic PTSA[14] to the synthesis of α,β-unsaturated thioesters from γ-sulfur substituted propargyl alcohols[15] to the rearrangement of 3-alkynyl-3-hydroxyl-1H-isoindoles in mildly acidic conditions to give the α,β-unsaturated carbonyl compounds.