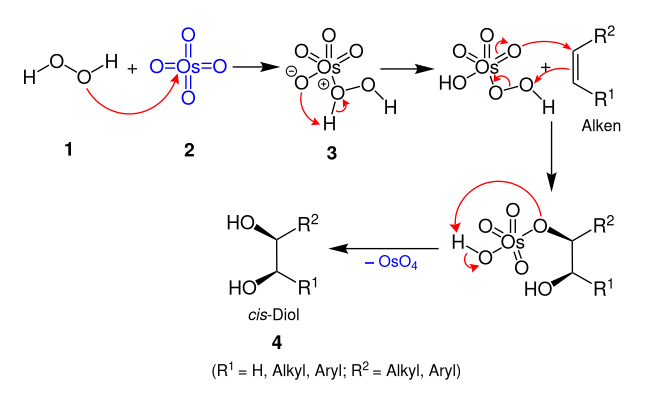
Milas hydroxylation
[1][2] The cis-diol is formed by reaction of alkenes with hydrogen peroxide and either ultraviolet light or a catalytic osmium tetroxide,[3] vanadium pentoxide, or chromium trioxide.
[2] One variation of the Milas hydroxylation (shown in the mechanism above) requires stoichiometric amounts of osmium tetroxide, which is toxic (highly volatile) and expensive.
[6] The catalyst, osmium tetroxide, also known as Merck osmic acid, dissolves readily in tertiary butyl alcohol which implies that the solution in which the reaction occurs is stable, unless isobutylene is already present.
In the presence of isobutylene most of the osmium tetroxide is reduced to an insoluble black colloidal oxide.
Hydrogen peroxide in tertiary butyl alcohol with osmium tetroxide as a catalyst (Milas reagents) was examined to determine the parallels of the reaction with butter yellow in vivo versus in vitro.